The particles of a liquid have 3 kinetic motion. The particles in a liquid are tightly packed together and move past each other allowing it to flow and because it flows Liquids does not have a definite shape it has a definite volume.

The Particulate Nature Of Matter Igcse Cambridge 2019 2021 Teaching Resources States Of Matter Matter Science Matter Worksheets
When the particles of a solid are heated they move faster move farther apart and take up more room.
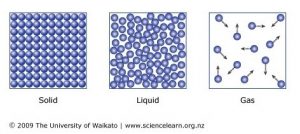
. How do particles in a gas move. They acquire the shape of the container in which they are. Describe the motion of particles in.
How do particles in a solid move. Discuss the processes by which vaporization and freezing occur. Held in fixed pattern.
Particle motion increases when temperature increases. When the particles of a solid are heated they move faster move farther apart and take up more room. How do we know particles are moving all the time.
Particles in a solid vibrate. Particles in liquid states have low kinetic energy meaning they also have a low temperature. Describe the motion of particles in a liquid when its heated.
Explain why a sewing needle can float on the surface of water in a glass. 3 Get Other questions on the subject. Particles in a liquid are more loosely packed and can flow around each other.
2 Show answers Another question on Chemistry. How do particles in the plasma state move. Describe the movement of particles in solid liquid gas and plasma states.
Describe the motion of particles in a liquid as it freezes. Vibrate about fixed positions. Vibrational rotational and translational.
Get started for FREE Continue. We know particles are moving because of diffusion. Particles in a solid are tightly packed and have limited movement.
Describe the motion of particles in solids and the properties of solids according to the kinetic-molecular theory. Danny Landon DestinyIanTyler How do particles in liquids move. Describe the energy and motion of particles in a solid.
Achange in the number of neutrons in an atom will change an blank. Chemistry 22062019 0400. When the particles of a liquid such as water are heated they move faster move farther apart and take up more room.
The attraction between each particle is weak enough for each to flow past one another but strong enough to hold their shape. Describe the motion of particles in liquids and the properties of liquids according to the kinetic-molecular theory. Can slide over each other.
You put in a 117 g ice cube at 000 c to form a system of ice original water. Distinguish between the two types of. All these motions apply in this scenario.
Describe the motion of particles in a liquid when its heated. 510 describe the arrangement and motion of particles in solids liquids and gases. When the particles of a solid are heated they move faster move farther apart and take up more room.
The specific heat of liquid water is 4190 jkgk. Describe the motion of particles in a liquid when its heated. And the heat of fusion of.
When the particles of a liquid such as water are heated they move faster move farther apart and take up more room. The particles of a liquid are able to move past each other. When the particles of a liquid such as water are heated they move faster move farther apart and take up more room.
When the number of protons changes in an atom a new element will form. So we can say that all particles show motion more or less. When the particles of a solid are heated they move faster move farther apart and take up more room.
Liquid state can be considered as an intermediate state of matter in which particles can move and do not have fixed position but their kinetic energy is less than gaseous particles and more than solid particles. Describe the energy and motion of particles in a solid. The motion of liquid particles.

5 10 Describe The Arrangement And Motion Of Particles In Solids Liquids And Gases Tutormyself Chemistry

Brownian Motion Easy Science Brownian Motion Interactive Science Notebook Motion

How Can You Describe The Motion Of Particles In A Solid How Can You Describe The Motion Of Particles In A Liquid How Can You Describe The Motion Of Particles Ppt
0 Comments